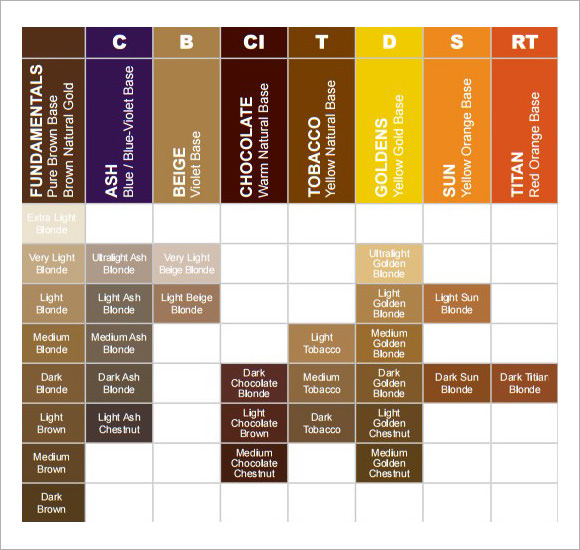
De Priester Charts Calculator online, free

Simple Calculator - A nice Simple Free Online Calculator. Easy to use and read. Online Abacus - An Online Abacus! Teach numbers from 1 to 50:-) Darts Calculator - Forget the maths, and play Darts! Maths Calculator - This Online Maths Calculator show the history of your sums. Stopwatch - Online Stopwatch, FULL SCREEN Stopwatch.
De Priester Charts Calculator Online Calculator

De Priester Charts Calculator Online
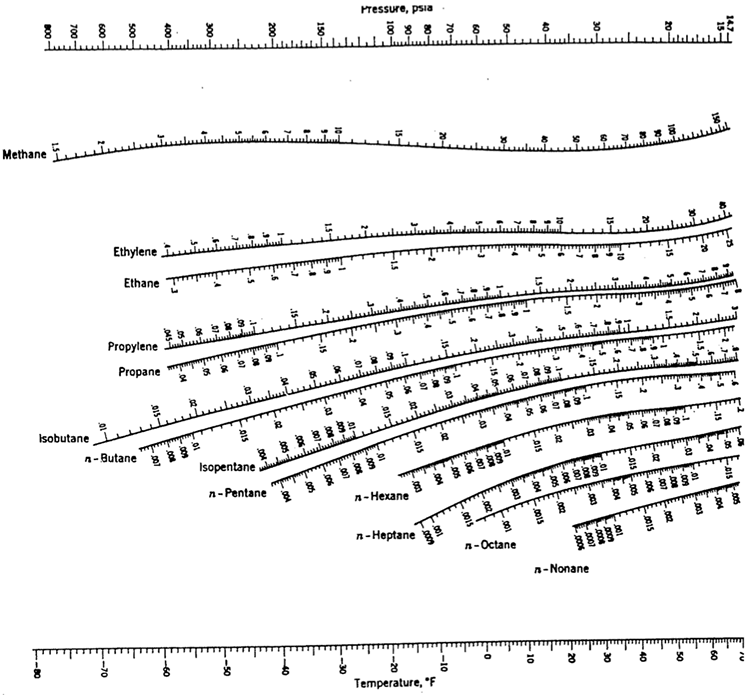
Here is an example of how to use a DePriester chart for propane at 70 psia and 20 °F: 1. Use the sliders to set the pressure and temperature. The green line connects these two points. The K-value is where the green line intersects the curve for propane (K = 0.8). De Priester Chart - Free download as PDF File (.pdf), Text File (.txt) or read online for free.
Depriester Chart Calculator Online
Simulations > Separations > DePriester Chart for Hydrocarbons
Details: Here is an example of how to use a DePriester chart for propane at 70 psia and 20 °F: 1. Use the sliders to set the pressure and temperature. 2. The green line connects these two points. 3. The K-value is where the green line intersects the curve for propane (K = 0.8). Reference: [1] J. M. Smith, H. C. Van Ness and M. M. Abbott,Introduction to Chemical Engineering Thermodynamics, 7th ed., Boston: McGraw-Hill, 2005. |